Catheter Medical Device Classification . this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr medical device classification is based on the device’s potential risk of harm to users. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu).
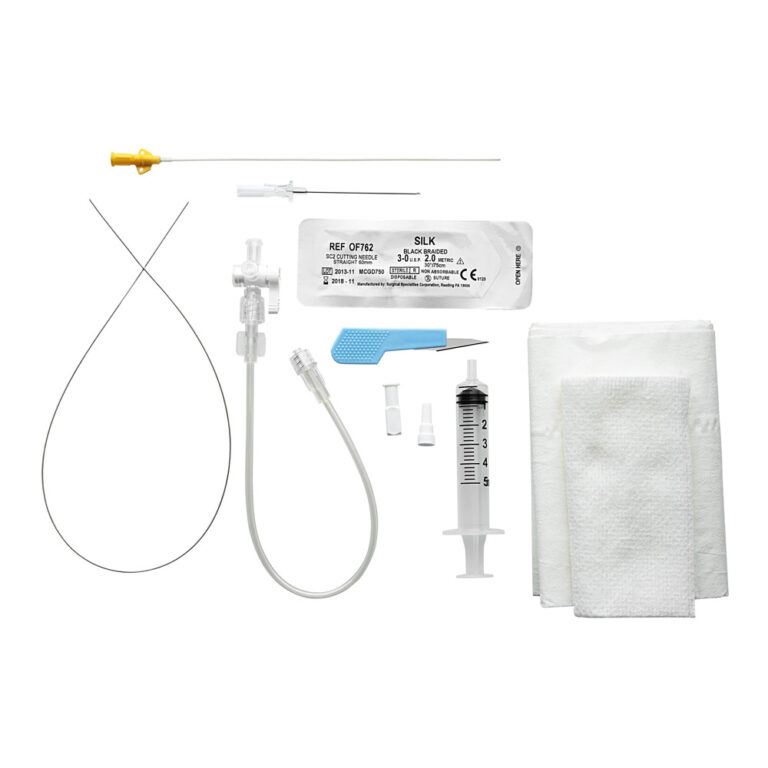
from www.argonmedical.com
learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr medical device classification is based on the device’s potential risk of harm to users. a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu).
Arterial Catheter Kits Argon Medical Devices
Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). a guidance document by the european commission on how to apply the classification rules for medical devices as.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Catheter Medical Device Classification the mdr medical device classification is based on the device’s potential risk of harm to users. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides. Catheter Medical Device Classification.
From mavink.com
Midline Catheter Types Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance. Catheter Medical Device Classification.
From mavink.com
Types Of Venous Access Devices Catheter Medical Device Classification learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr. Catheter Medical Device Classification.
From www.vrogue.co
What Are The Different Types Of Central Venous Cathet vrogue.co Catheter Medical Device Classification this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). this document provides guidance on how to classify medical devices based on their intended use and risk. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. the. Catheter Medical Device Classification.
From www.mentice.com
Understanding Sheaths and Catheters Mentice Blog Catheter Medical Device Classification the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance. Catheter Medical Device Classification.
From getcompletecare.com
Catheters 101 The Basics of Urinary Catheter Types Catheter Medical Device Classification learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance document by the european commission on how to apply the classification rules for medical devices as. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides. Catheter Medical Device Classification.
From www.hcd.com
Types of Catheters Uses, Styles, and How to Get Them HCD Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. a guidance document by the european commission on how to apply the classification rules for medical devices as. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. this document. Catheter Medical Device Classification.
From finneganmedicalsupply.com
Catheter 101 Different Types of Catheters for Urinary Incontinence Catheter Medical Device Classification this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr medical device classification is based on the device’s potential risk of harm to users. learn how the fda. Catheter Medical Device Classification.
From www.ontariorenalnetwork.ca
Types of Body Access for Dialysis ORN Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). the mdr medical device classification is based on the device’s potential risk of harm to users. a guidance document by. Catheter Medical Device Classification.
From surgicaltechie.com
Foleys Catheter uses and Types Uses and different sizes Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance. Catheter Medical Device Classification.
From hmdhealthcare.com
BlogImages2_UnderstandingTheTypesOfCatheters,TheirUses,And Catheter Medical Device Classification a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). the mdr medical device classification is based on the device’s potential risk of harm to users. learn how the. Catheter Medical Device Classification.
From smartstuff.howstuffworks.com
Understanding the Types and Uses of Catheters HowStuffWorks Catheter Medical Device Classification learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). . Catheter Medical Device Classification.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Catheter Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). a guidance document by the european commission on how to apply the classification rules for medical devices as. the mdr. Catheter Medical Device Classification.
From www.consuremedical.com
What Are the Best Male External Catheters? Consure Medical Catheter Medical Device Classification a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. . Catheter Medical Device Classification.
From www.argonmedical.com
Arterial Catheter Kits Argon Medical Devices Catheter Medical Device Classification learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. this document provides guidance on how to classify medical devices based on their intended use and risk. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance. Catheter Medical Device Classification.
From stock.adobe.com
Tunneled and nontunneled central venous catheters placed in the Catheter Medical Device Classification the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). a guidance document by the european commission on how to apply the classification rules for medical devices as. this document provides. Catheter Medical Device Classification.
From www.unitekcollege.edu
A StepbyStep Guide to Catheterization Unitek College Catheter Medical Device Classification a guidance document by the european commission on how to apply the classification rules for medical devices as. learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. this document provides guidance on how to classify medical devices based on their intended use and risk. this document. Catheter Medical Device Classification.
From mavink.com
Urinary Catheter Sizes Catheter Medical Device Classification learn how the fda classifies medical devices into three regulatory classes based on risk, intended use, and indications for. the mdr medical device classification is based on the device’s potential risk of harm to users. this document provides guidance on the codes for the designation of notified bodies in medical devices under regulation (eu). a guidance. Catheter Medical Device Classification.